Beta Decay follow-up applet
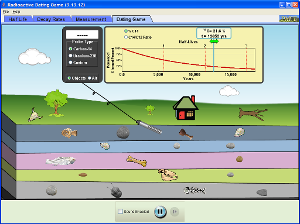
1. Your goal is to date 10 items on the simulation below. Tell me how you found each one, making a table
|ITEM | ISOTOPE USED | ESTIMATED AGE |
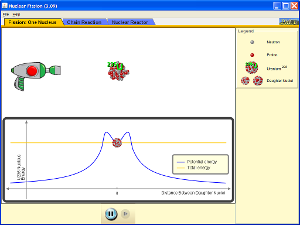
Run the simulation. Before the gun is fired, is the material stable, or does it seem likely to radioactively decay?
When you operate the gun, what type of particle does it fire?
If the gun fires and “hits” the nucleus, what happens?
Switch the tab to “chain reaction” and add some uranium-238. The atom used in the previous tab was uranium-235. Is uranium-238 “fissionable”? How does firing the gun on a uranium-238 atom change it? (Note you can aim the gun.)
Chain reaction:
Switch to the tab “Chain Reaction”.
Reset the sim (using the button) and add lots of fissionable uranium-235. What happens and why?
Normal levels of the uranium-235 isotope are about 0.72%, with the majority being uranium-238. Round the level up to 1% (in the favor of uranium-238), to one atom of uranium-235 and 99 atoms of uranium 238. Use the simulation to discover if naturally-derived uranium can start a chain reaction (and therefore be useful in either nuclear weapons or nuclear power plants): Is naturally derived uranium able to start a chain reaction, or must the sample be “enriched”?
Use the simulation to find a minimum ratio of uranium-235 to uranium-238 (keep your total of atoms always at 100); what is the smallest percentage that still starts a chain reaction?
How does the above compare to “weapons-grade” enriched uranium (about 80-85%)?
(these questions mirrored from here)
| Click to Run |
| Click to Run |
This is just the information I am finding everywhere. Thanks for your blog, I just subscribe your blog. This is a nice blog.. dating sites
ReplyDelete