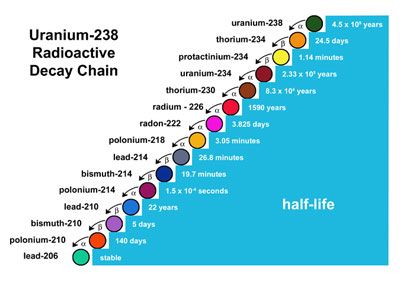
"What does this radioactivity stuff have to do with physics anyway?"
Great question.
Physics is the study of forces, energy and motion. There are four main forces in the universe, according to current theory
MACRO FORCES
Gravity (the weakest force)
Electromagnetism (aspects of which include all the forces we study in the rest of the class)
NUCLEAR FORCES
Weak force (also known as radioactivity)
Strong force (the holds together the pieces that make up protons and neutrons)
We've started out this class with a snippet of information about Nuclear Forces, and next week, we move on to Macro Forces for the rest of the course. Before we do that, two things need to be accomplished.
INDIVIDUALLY complete the work shown here
Create a public service announcement about a radioactive application using one of the following isotopes. One common application of the isotope is shown in ( ). Use the process described in
http://www.mindspring.com/~mmm/element.html to communicate your ideas. You must include a written plan with your video that addresses these components and answers the questions below.
- (irradiation of spices)cobalt-60
- Cs-133 (atomic clock)
- Iodine-131
- Americium-241 (gauging plastic)
- Americium-241 (smoke detectors)
- (CT or PET scans ) C-11, N-13, or O-15
- Technicium-99m
- (Fresh food irradiation) cobalt-60
- Leakage from Fukoshima (caesium or cesium contaminated water)
- C-14 (radioactive dating)
- Some other isotope of your choice
The following information must be present in your background information:
a. What type of decay is going on?
b. What is the half-life of the isotope, and how long will it take for 99% to disappear
c. How can we protect humans from this type of isotope when we don't want to be exposed to it?
d. How are force or momentum used in this process?
e. How valuable is this process?
f. How is the use of this radioactive tool comparable to a non-nuclear option. Pick two similarities and two differences.
g. Why is radioactivity all around us? And what's the difference between natural radioactivity and deadly radioactivity.
Upload your video to a youtube account and share with me.
Grading Rubric
Background notes and content correctness: 10 points
Entertainment value: 10 points
Use of media to enhance presentation: 10 points
Involvement by all members of group: 10 points
Title or Credits, as appropriate: 5 points